A Science-driven Wellness Journey to
FIND A
NEW YOU
Doctor-led programs that empower holistic wellness at the intersection of ancient and modern healing approaches, to help unlock new energy, resilience, and well-being.
THE JOURNEY
STARTS WITH YOU
At Soul Spring, we believe that wellbeing is about more than solely physical health. Our technology-led, bio-individualized wellness protocols help you live a fulfilling life and find a new you.
Whether you walk into a city clinic, retreat, or resort – our certified health and wellness practitioners are present to guide you in your journey towards holistic wellbeing.
THE SOUL SPRING
METHOD
THE SOUL SPRING DIFFERENCE,
AT YOUR CONVENIENCE

Soul Spring Oasis has been designed keeping every aspect of holistic wellness in mind.
Our wellness centers are strategically located, in the midst of the city and busy life



Soul Spring Oasis has been designed keeping every aspect of holistic wellness in mind.
Our wellness centers are strategically located, in the midst of the city and busy life


GUIDED BY
INNOVATION
At Soul Spring, we have our own medical R&D team comprising dedicated groups of doctors and med tech researchers. This allows us to create a strong technological backbone for our wellness protocols and ensure you find a new you, at a fundamental level.
We also leverage FDA-registered devices from our sister company Wegamed, Germany and other leading med tech companies to offer you empirical evidence of the connection between underlying physiological and psychological conditions, and the apparent symptoms.

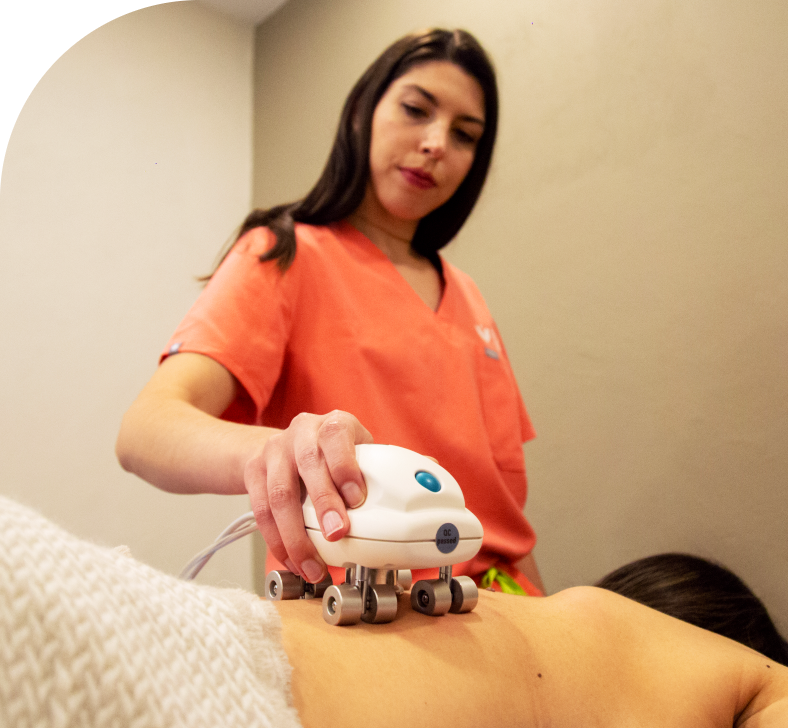
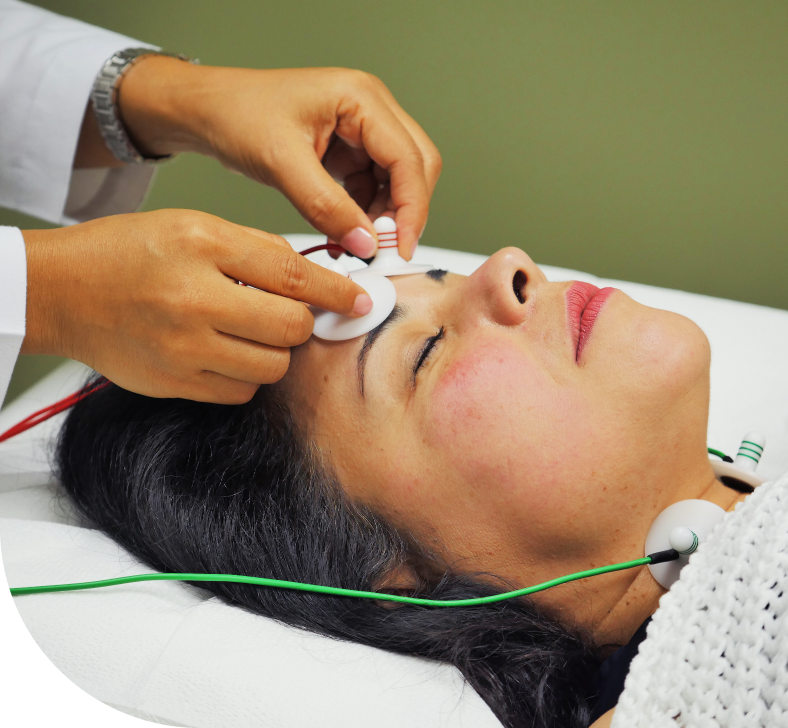
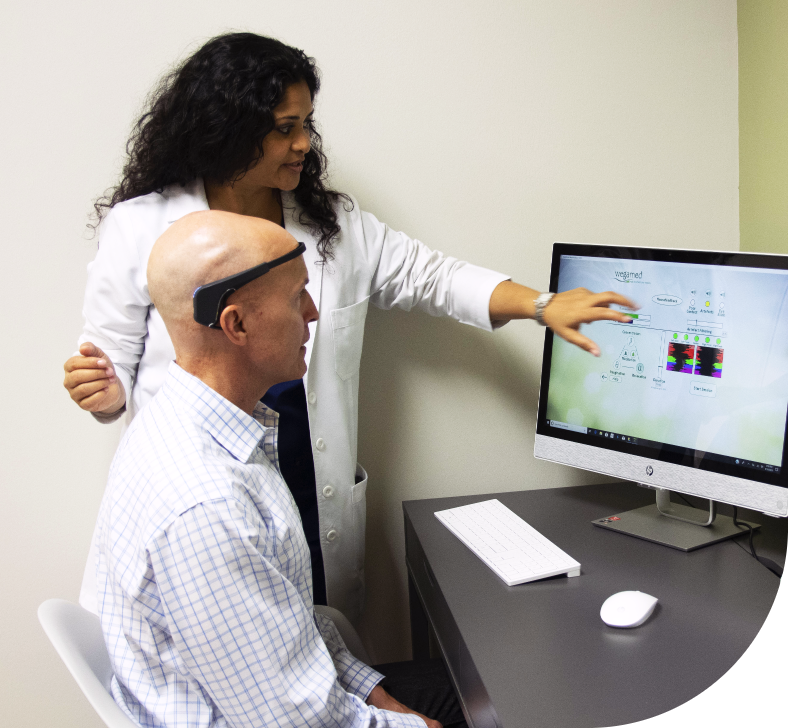




OUR CENTERS
AT A GLANCE



SOUL SPRING CUERNAVACA
Hotel Compañíade Especias Avenida, Club de Golf Santa Fe 1. Xochitepec Morelos


SOUL SPRING LOMAS
Pedregal 51, Lomas – Virreyes, Lomas de Chapultepec IV Secc Miguel Hidalgo, 11000 Ciudad de México, CDMX
SOUL SPRING WOODLANDS
25420 Kuykendahl Rd, Suite D100, Tomball, TX, 77375
SOUL SPRING CHICAGO
1780 Green Bay Rd, Highland Park, IL 60035, USA
SOUL SPRING CUERNAVACA
Club de Golf Santa Fe 1. Xochitepec Morelos
SOUL SPRING HUMBLE
9701 North Sam Houston Pkwy E. Suite #250 Humble, TX, USA
SOUL SPRING LOMAS
Pedregal 51, Lomas – Virreyes, Lomas de Chapultepec IV Secc Miguel Hidalgo, 11000 Ciudad de México, CDMX
SOUL SPRING WOODLANDS
SOUL SPRING LOMAS
SEEN, HEARD & FEATURED


UNPARALLELED
EXPERIENCES
Soul Spring helped me understand how my emotions affect my well-being. I enjoy every Soul Spring session, not only the moment, but the whole process, the experience, and above all, the result.
Marcela Castellanos

Soul Spring has been a unique space where I have been able to reconnect with myself and find healing for my mind and body.
Marcos Oteiza

Soul Spring has returned vitality and absolute health to me. I feel a great change in my energy and ability in all areas of my life.
Francesca O.

For me, Soul Spring has been an unexpected and very exciting discovery. Thanks to Soul Spring, my body’s capacity to heal itself has improved and my inner strength has been regenerated.
Antonio Cervantes

Everything about my health is much better now. I have more energy and focus, with no aches and pains.
Carlos Rojas

There is no place on earth like it and it is a lens on the future, not only for our health but for the health of the bio-wellness industry.
Grant Hunter

Soul Spring helped me understand how my emotions affect my well-being. I enjoy every Soul Spring session, not only the moment, but the whole process, the experience, and above all, the result.

Marcela Castellanos
Soul Spring has been a unique space where I have been able to reconnect with myself and find healing for my mind and body.

Marcos Oteiza
Soul Spring has returned vitality and absolute health to me. I feel a great change in my energy and ability in all areas of my life.

Francesca O.
For me, Soul Spring has been an unexpected and very exciting discovery. Thanks to Soul Spring, my body’s capacity to heal itself has improved and my inner strength has been regenerated.

Antonio Cervantes
Everything about my health is much better now. I have more energy and focus, with no aches and pains.

Carlos Rojas
There is no place on earth like it and it is a lens on the future, not only for our health but for the health of the bio-wellness industry.

Grant Hunter
FIND THE
NEW YOU
HABLE CON UN EXPERTO

THE SOUL BOOK
A resource center
Dive into the Soul Spring blogs and discover what we can do for your health
THE SOUL BOOK
A Resource center

Dive into the Soul Spring blogs and discover what we can do for your health
